Background: Diffuse Large B-Cell Lymphoma (DLBCL) is the most common B-cell non-Hodgkin lymphoma. DLBCL commonly affects patients with comorbidities and the elderly. To date, chemoimmunotherapy remains the standard of care for these patients. Data on patients aged ≥65 years managed in Latin America (LATAM) are scarce. Herein, we examine the clinical, treatment and outcome patterns in older patients with DLBCL, with a focus on the effect of treatment in survival outcomes.
Methods: We retrospectively analyzed patients aged ≥65 years with newly diagnosed DLBCL managed at different academic institutions in 8 LATAM countries. Demographic characteristics are reported using descriptive statistics. All patients received treatment; those who completed 6 cycles and underwent either CT or PETCT at the end of therapy were considered evaluable for response. Survival curves were estimated using the Kaplan-Meier method. Logistic and Cox proportional-hazard regression models were used to evaluate parameters associated with response and survival.
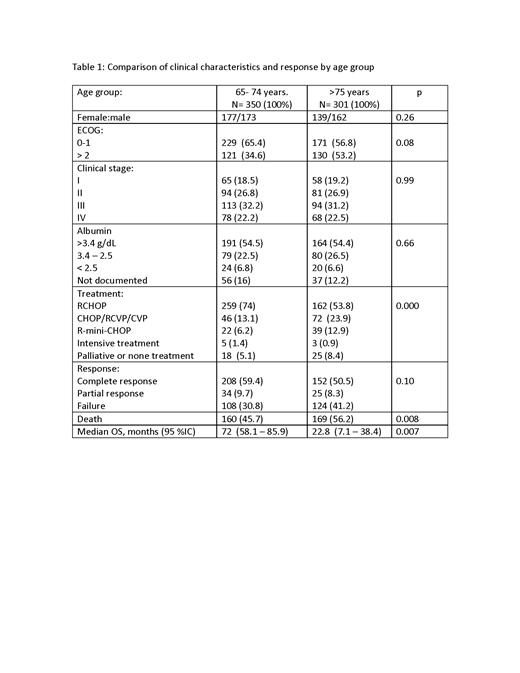
Results: A total of 651 patients were identified and had sufficient data for analysis; median age was 74.1 years (65-96), 335 (51.5%) were female, 400 (61.4%) had ECOG ≤1, and 426 (65.4%) had advanced disease (stage III-IV). No difference in clinical features and laboratory parameters were identified among patients aged <75 vs ≥75 years (Table). The most common first-line chemoimmunotherapy regimens used were standard dose R-CHOP (n=421, 64.7%), R-CVP (n= 74, 11.4 %), R-mini-CHOP (n= 61, 9.4%), and CHOP (n= 29, 4.5%). Eight patients (1.2%) received R-EPOCH, and 43 (6.6%) best supportive care including some receiving dexamethasone and/or rituximab monotherapy. In all patients, the overall response rate was 64.4% (complete 55.3%, partial 9.1%). Responses could not be evaluated in 219 cases (33.6%) either because of early death secondary to toxicity or lymphoma progression during therapy (n=212, 32.5%), or as a complication of patients' comorbidities (n=16, 2.5%). With a median follow up of 60 months (range 1-100), the median overall survival (OS) time was 56 months (95% CI: 38.7-73.2) with a 5-year OS rate of 50%. The causes of death were lymphoma (n=180, 27.6%), infection (n=72,11.1%), non-infectious toxicity (n=6, 0.9%), ischemic cardiopathy (n=7, 1.1%), heart failure (n=6, 0.9%), cerebrovascular event (n=6, 0.9%), and unknown in 41 cases (6.3%). Given our prior work on the impact of serum albumin in DLBCL patients, we analyzed serum albumin levels in elderly patients with DLBCL. Hypoalbuminemia (albumin <3.5mg/dL) was associated with a higher frequency of advanced disease (n=211, 37.8% vs n=152, 27.2%; [p=0.005]), high-risk IPI score (n=77, 14.7% vs n=62, 11%; [p=0.000]), and death (n=147, 22.5% vs n=128, 19.6%; [p=0.000]). In the univariate analysis, the factors influencing mortality were: ECOG ≤1 (p=0.000, HR 1.25[95% IC: 1.0-1.4]), hypoalbuminemia (serum albumin <3.5mg/dL; p=0.031, HR 1.24 [95% IC: 1.02 -1.51]), the use of anthracyclines (p=0.002, HR 0.63, [95 % IC: 0.47 -0.84]), and no achieving a complete response (CR) to first-line treatment (p=0.000, HR 5.01 [95 %IC: 4.25 -6.0]. In the multivariate analysis, all factors remained statistically significant: ECOG ≤1 (p=0.000, aHR 1.32[95 IC: 1.1 -1.5]), hypoalbuminemia (p=0.005, aHR 1.33 [95 % IC: 1.08 -1.6]), the use of anthracyclines (p=0.006, aHR 0.66 [95 % IC: 0.49 -0.88]), and no achieving CR to first-line treatment (p=0.000, aHR 4.91 [95 %IC: 4.1 -5.7].
Conclusions: To our knowledge, this is one of the largest real-world studies on elderly patients with DLBCL in LATAM. In this cohort, survival rates were comparable to those previously reported in the literature. This statement was also true when evaluating the response rates to first-line therapy. We found that ECOG performance status, serum albumin levels, the use of anthracycline and response to first-line therapy were all independently associated to survival. Although the main limitation of this study is its retrospective design, we report that current practices in LATAM remain appropriate for our context and known limitations, thus, this study provides some evidence to clinicians on the therapy patterns and outcomes of such therapies in elderly patients with DLBCL living in resource-limited settings.
Disclosures
Villela Martinez:roche: Speakers Bureau; Merck Sharp and Dome: Speakers Bureau; astra zeneca: Speakers Bureau; TEVA: Speakers Bureau; Sanofi: Speakers Bureau. Ramirez:merck sharp and dome: Speakers Bureau; roche: Speakers Bureau. Quintero:Merck Sharp and dome: Speakers Bureau; Takeda: Speakers Bureau; astra zeneca: Speakers Bureau; roche: Speakers Bureau. Perini:MSD: Consultancy, Speakers Bureau; Merck: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astra zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Lilly: Consultancy, Speakers Bureau. Peña:Janssen: Other: Congress Travel expenses. Gomez-Almaguer:AMGEN: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Novartis: Honoraria; AbbVie: Consultancy, Honoraria. Castillo:Loxo: Consultancy, Research Funding; Kite: Consultancy; Pharmacyclics: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Mustang Bio: Consultancy; Cellectar: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal